
For this reason, patients with a measured serum aluminum concentration >200 mcg/L should not be treated with deferoxamine as it may lead to severely high levels of aluminum and fatal neurotoxicity. Deferoxamine can draw aluminum deposited in tissues into the plasma. In the case of CKD/ESRD patients, the product is dialyzable using a high-flux membrane.
#IRON TOXICITY ANTIDOTE FREE#
If chelation occurs in hepatocytes, the compound will be excreted in bile, and when chelation occurs with free iron in plasma or other tissues, it is excreted by the kidneys.ĭeferoxamine can also bind aluminum within the plasma to form aluminoxane, which is renally excreted. When bound, the resultant ferrioxamine is very water-soluble. Although this is a small fraction of total body iron, it has a profound effect. Thus, only a small amount of iron is available for chelation at any given time. Although deferoxamine can directly bind and remove iron from myocardial cells, it will not bind iron already bound to molecules such as transferrin, hemoglobin, or cytochromes. Deferoxamine chelates non-transferrin bound iron (free iron), iron in transit between transferrin and ferritin (labile chelating iron pool), hemosiderin, and ferritin. The bound form of deferoxamine is then excreted via the urine or bile. deferoxamine is a hexadentate molecule and is able to bind iron at a 1-to-1 ratio. It binds free plasma iron and excess iron within cells.

Deferoxamine is a molecule produced by the fermentation of Streptomyces pilosus. Free iron also precipitates acidosis by inhibiting oxidative phosphorylation in mitochondria. Free radicals lead to DNA destruction and cellular damage. This excess iron within cells catalyzes the production of free radicals via the Fenton reaction. Ĭells also take up free iron to form labile iron pools (LIP) within their cell membranes. These include non-transferable bound iron (NTBI) and labile plasma iron (LBI). When iron storing proteins become saturated, free iron species accumulate in the plasma. Instead, humans regulate GI uptake by altering hepcidin levels. No physiological mechanism exists to excrete iron. It is stored by ferritin and transferred throughout the serum by transferrin. It is a vital element in proteins such as hemoglobin, myoglobin, and cytochrome and also functions as a cofactor for many enzymes. Iron is an essential part of human physiology.
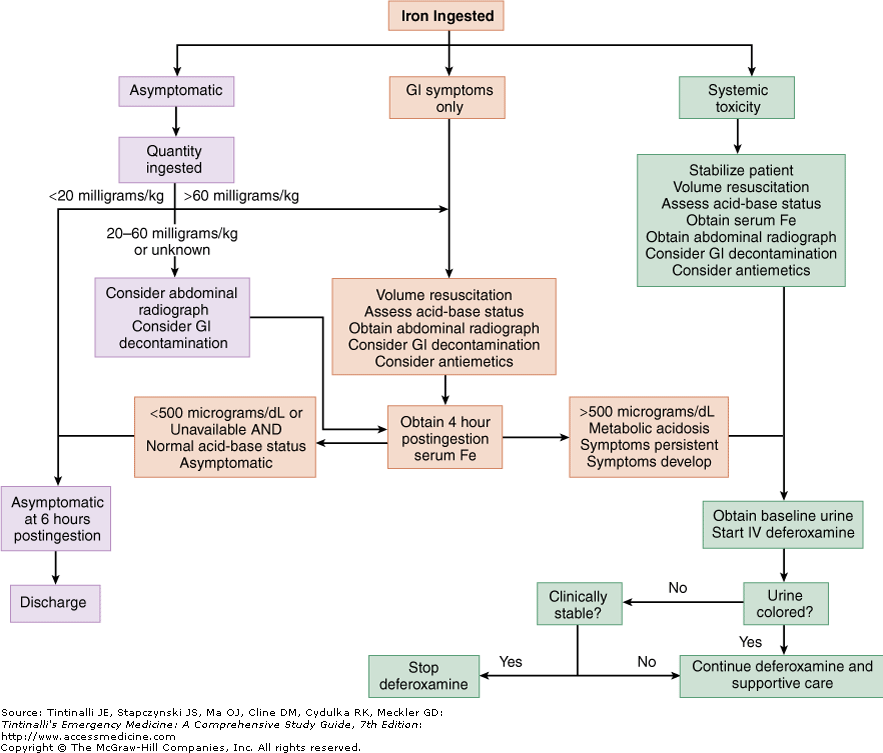
It is also an indicated use in acute toxicity, which results from exposure to >200 mcg/L of aluminum resulting in acute encephalopathy. Its use is indicated in patients with signs and symptoms associated with chronic aluminum levels greater than >20 mcg/L, such as osteomalacia, anemia, hypercalcemia, and dialysis dementia. Aluminum toxicity can occur in patients with chronic kidney disease who undergo bladder irrigation with aluminum-containing products, use phosphate binders that contain aluminum, or receive hemodialysis with a water source contaminated with aluminum. Thus, rapid progression through these stages is another indicator that chelation may be necessary.Īluminum toxicity is an off-label use for deferoxamine chelation therapy. Acute iron toxicity progresses through five clinical stages, with the most deadly consequences occurring within the first four days. Signs and symptoms of systemic toxicity include coagulopathy, cardiomyopathy, and hepatic and renal failure.


Īnother indication for iron chelation is a cardiac T2* 500 mcg/dL are considered hazardous, and chelation should be initiated. In this population, chelation should begin two years after transfusions start, serum ferritin levels greater than 1000 mcg/L, or when liver iron concentration (LIC) is greater than 3 mg Fe/g. These patients include those affected by Thalassemia, Sickle cell disease, myelodysplastic syndromes, ineffective hematopoiesis, and other inherited anemic disorders. Transfusion-related iron overload occurs in patients that require frequent transfusions throughout their life. Clinicians can also use deferoxamine as an off-label treatment for aluminum toxicity in chronic kidney disease (CKD) patients. The FDA has not approved deferoxamine as first-line therapy for hereditary hemochromatosis unless there is a contraindication to phlebotomy.
#IRON TOXICITY ANTIDOTE SERIAL#
The definition of iron overload is serial ferritin levels above 800 to 3000 ng/mL. Deferoxamine (DFO or DFOA) is FDA approved to treat iron overload, either acute or chronic.


 0 kommentar(er)
0 kommentar(er)
